RFP & CER Creation process
1. Proposal Creation Process:
- Understanding Client Needs: Start by thoroughly understanding the client’s requirements and objectives. This involves close communication with the client to grasp their expectations and desired outcomes.
- Team Collaboration: Engage in collaborative efforts within the consultancy team. Leverage the expertise of various team members to ensure a well-rounded and comprehensive proposal.
- Proposal Writing: Develop a clear and compelling proposal that outlines the consultancy’s approach, methodology, timeline, deliverables, and pricing. Pay attention to language, formatting, and presentation to make the proposal visually appealing and easy to understand.
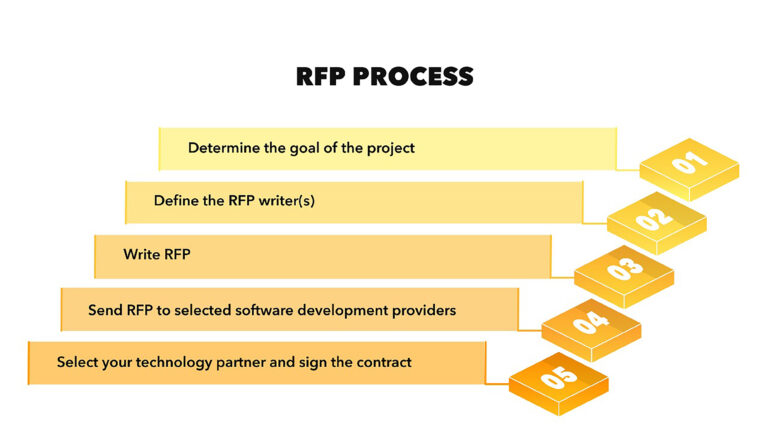
2. RFP Response Process:
- RFP Analysis: Carefully analyze the RFP document to understand the client’s requirements, evaluation criteria, and submission guidelines. Identify key deadlines and any specific instructions provided.
- Resource Allocation: Determine the resources required for crafting a competitive RFP response. This includes assembling a dedicated team with the necessary skills and expertise.
- Content Development: Develop a tailored response addressing each section of the RFP. Highlight the consultancy’s strengths, relevant experience, and unique value proposition. Ensure compliance with all requirements specified in the RFP.
- Quality Assurance: Conduct thorough reviews and quality checks to eliminate errors, inconsistencies, and ensure that the response aligns with the client’s needs.
3. CER Creation Process:
- Regulatory Compliance: Understand and adhere to regulatory requirements for Clinical Evaluation Reports, especially in industries such as healthcare and life sciences.
- Data Collection: Gather relevant clinical data, including information on the safety and performance of products or services. This may involve collaboration with subject matter experts, researchers, or other stakeholders.
- Literature Review: Conduct a comprehensive literature review to incorporate existing scientific knowledge and findings related to the products or services under evaluation.
- Report Writing: Develop a well-structured Clinical Evaluation Report that presents the collected data, analysis, and conclusions in a clear and concise manner. Ensure that the report meets regulatory standards and guidelines.
4. Document Management:
- Version Control: Implement a robust version control system to track changes and updates to proposals, RFP responses, and CERs.
- Secure Storage: Safely store all documents, ensuring that they are easily accessible to the relevant team members while maintaining confidentiality and security.
